Our vision
Accelerating the impact of life-changing therapies worldwide.
Early Phase Services
At Syneos Health, our Early Phase team has over 30 years of experience running safe, high-quality clinical studies.
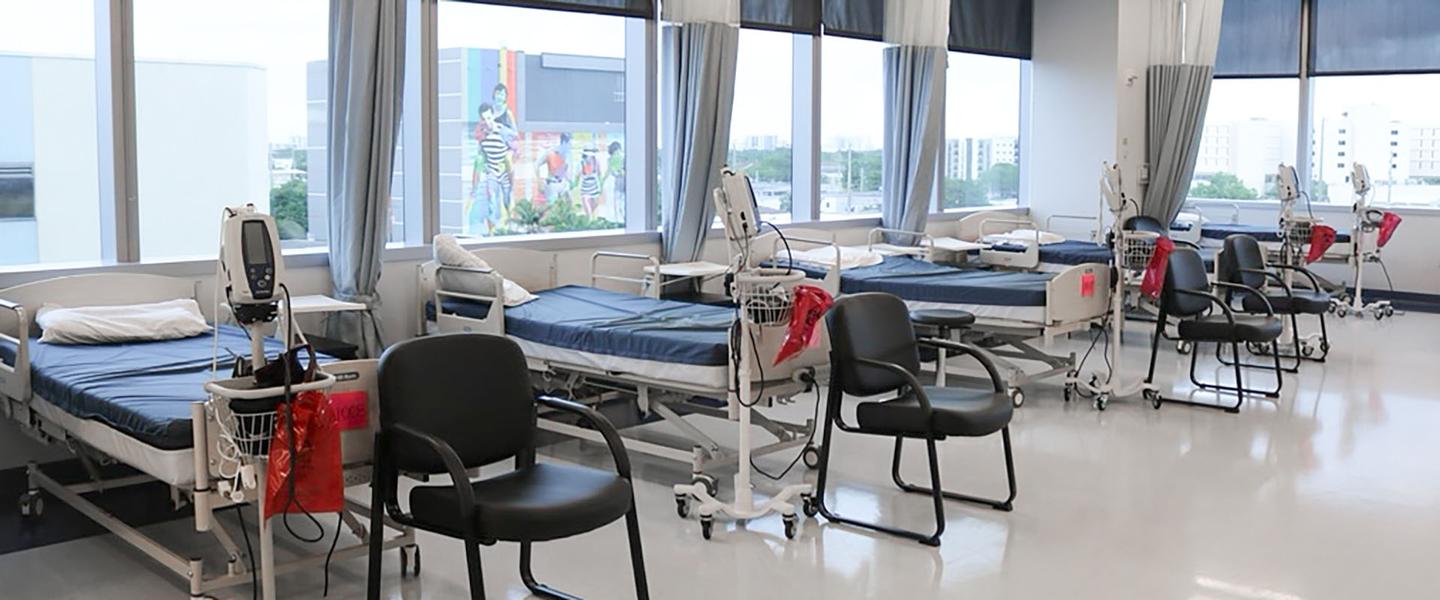
We operate advanced research units in Quebec City and Miami, staffed by more than 800 medical and scientific professionals dedicated to participant care.
Our facilities are designed to conduct complex studies while keeping your comfort and safety at the center of everything we do.

Syneos Health by the Numbers
-
0 Number of client partners
-
0 Number of disease categories
-
0 Number of employees
-
0 Years as a global company
-
0 Number of novel drugs approved by the FDA
-
0 Number of projects delivered
Drug development
Clinical studies are usually conducted in several phases in a gradual and safe manner. At Syneos Health, we conduct bioequivalence studies (on generic drugs) as well as phase I and IIa studies.
Pre-Clinical
- Goal: Test safety in lab and animals before human trials.
- Participants: No humans (lab and animal studies).
- Timeline: Months to years.
Phase I
- Goal: Check safety and how the drug is processed by the body.
- Participants: 20–100 healthy volunteers or patients.
- Timeline: ~6–12 months.
Phase II
- Goal: See if the treatment works and monitor side effects.
- Participants: 100–300 patients.
- Timeline: ~1–2 years.
Phase III
- Goal: Confirm effectiveness and compare with standard treatments.
- Participants: 1,000–3,000 patients.
- Timeline: ~1–4 years.
Phase IV
- Goal: Monitor long-term safety and real-world use.
- Participants: Tens of thousands (general public).
- Timeline: Ongoing.

 Skip site navigation
Skip site navigation